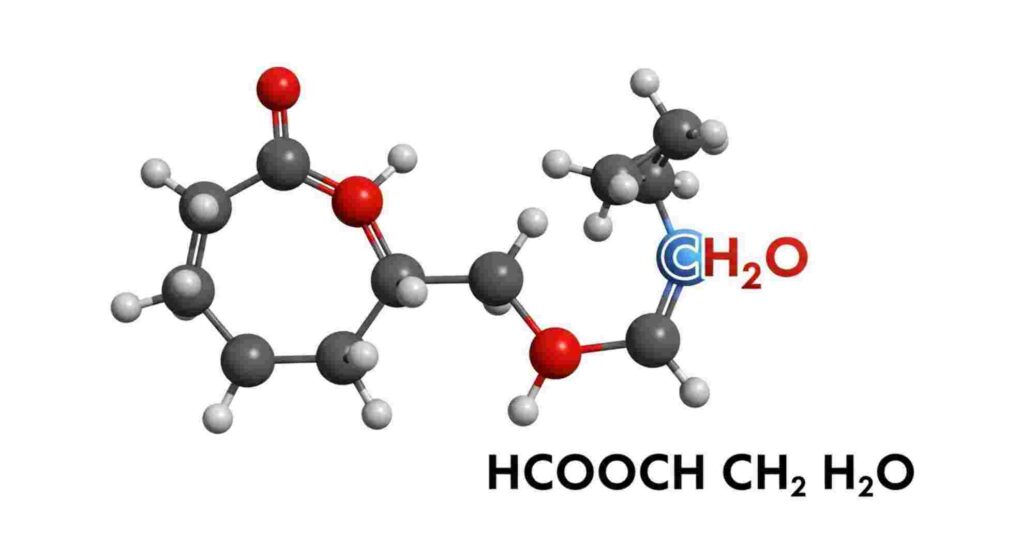
In the world of chemistry, understanding molecular structures and their functions is fundamental. One intriguing compound that captures the curiosity of scientists and students alike is HCOOCH CH2 H2O. This chemical formula represents a specific organic molecule with notable properties and potential applications. Whether you’re a budding chemist, a researcher, or simply someone interested in chemical compounds, this guide will walk you through everything you need to know about HCOOCH CH2 H2O.
What Is HCOOCH CH2 H2O?
HCOOCH CH2 H2O is a chemical structure that combines elements of organic chemistry with water molecules, indicating it could be a form of ester or related compound. Breaking down the formula:
- HCOO: This represents a formyl or formate group, which is common in organic acids and esters.
- CH: A carbon-hydrogen group, potentially part of a larger chain.
- CH2: A methylene group, often serving as a bridge in organic molecules.
- H2O: Water molecule, which might indicate hydration or a hydrate form of the compound.
The exact structure suggests that HCOOCH CH2 H2O is an ester derivative with water attached or incorporated, possibly a hydrate or a related compound.
Structural Details of HCOOCH CH2 H2O
Understanding the molecular structure is critical for predicting its behavior and applications. Based on its formula, HCOOCH CH2 H2O likely possesses:
- A formate ester backbone (HCOO-): common in various organic synthesis processes.
- An alkyl chain: represented by CH and CH2, indicating a chain or ring structure.
- A water molecule: either as an attached hydrate or part of the crystal lattice in solid form.
This combination suggests that HCOOCH CH2 H2O could be a crystalline hydrate of an ester, with water molecules bound within its structure, influencing its physical properties like melting point and solubility.
Chemical Properties of HCOOCH CH2 H2O
Solubility
One of the key features of compounds containing water of hydration is their solubility. Typically, hydrates display increased solubility in water compared to their anhydrous counterparts. For HCOOCH CH2 H2O, the presence of water molecules suggests it may be soluble in polar solvents like water and ethanol, making it versatile in various chemical reactions.
Melting and Boiling Points
Hydrated compounds often have distinct melting points. The water molecules stabilize the structure, often resulting in higher melting points than anhydrous forms. Precise data would depend on the specific structure, but expect HCOOCH CH2 H2O to melt at a temperature characteristic of its hydrate form.
Reactivity
As an ester derivative, HCOOCH CH2 H2O can undergo typical reactions associated with esters, such as hydrolysis to produce formic acid and alcohol. Its water content might influence reaction pathways, making it suitable for specific synthetic applications.
Applications of HCOOCH CH2 H2O
While HCOOCH CH2 H2O is not a widely known commercial compound, compounds with similar structures find diverse applications:
1. Organic Synthesis
Esters like HCOOCH CH2 H2O are valuable intermediates in organic synthesis. They can undergo hydrolysis, transesterification, and other reactions to produce useful chemicals, pharmaceuticals, or polymers.
2. Pharmaceutical Industry
Hydrated esters often have unique bioavailability or stability characteristics, making them candidates for drug development or delivery systems.
3. Material Science
Crystalline hydrates are studied for their physical properties, potential use in controlled-release systems, or as components in crystalline materials.
4. Research and Development
Academic and industrial research often investigates such compounds to understand their properties, stability, and potential new applications in green chemistry or sustainable materials.
How Is HCOOCH CH2 H2O Synthesized?
Synthesizing compounds like HCOOCH CH2 H2O typically involves esterification reactions, hydration processes, or crystallization techniques. Here’s a general overview:
- Ester formation: Reacting formic acid or formate salts with appropriate alcohols under acidic conditions.
- Hydration: Exposing the ester to water under controlled conditions to form the hydrate.
- Crystallization: Purifying the compound by crystallization from suitable solvents to obtain the hydrated form.
Precise synthetic routes depend on the desired purity, yield, and specific application.
Safety and Handling of HCOOCH CH2 H2O
As with all chemicals, safety precautions are essential:
- Handling: Use gloves and eye protection when working with esters and hydrates.
- Storage: Store in airtight containers away from heat and moisture.
- Disposal: Follow local regulations for chemical waste disposal, ensuring environmentally safe practices.
Due to limited data on this specific compound, consulting Material Safety Data Sheets (MSDS) for similar esters is advisable.
Future Perspectives and Research Opportunities
The unique structure of HCOOCH CH2 H2O opens doors for potential research in:
- Developing new biodegradable polymers.
- Designing novel drug delivery systems.
- Exploring its role in green chemistry as a renewable feedstock.
Further research can uncover more about its stability, reactivity, and environmental impact.
Conclusion
HCOOCH CH2 H2O is a fascinating chemical compound with a likely ester structure and water of hydration that influences its physical and chemical properties. Its potential applications span from organic synthesis to materials science and pharmaceuticals. As research advances, compounds like this could play significant roles in developing sustainable and innovative chemical solutions.
Understanding its structure, properties, and synthesis is essential for chemists seeking to harness its capabilities effectively. Whether you’re a student, researcher, or industry professional, exploring such compounds broadens your knowledge and opens new avenues for scientific discovery.